Why Is Ni Dmg 2 Insoluble In Water
- Why Is Ni Dmg 2 Insoluble In Water System
- Ni Dmg 2 Complex
- Why Is Ni Dmg 2 Insoluble In Water Heater
- Why Is Ni Dmg 2 Insoluble In Water Cycle
| Names | |
|---|---|
| IUPAC name | |
| Other names Nickel hydroxide, Theophrastite | |
| Identifiers | |
| |
| ChemSpider | |
| ECHA InfoCard | 100.031.813 |
| EC Number |
|
| RTECS number | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| Ni(OH)2 | |
| Molar mass | 92.724 g/mol (anhydrous) 110.72 g/mol (monohydrate) |
| Appearance | green crystals |
| Density | 4.10 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) (anhydrous, decomposes) |
| 0.13 g/L | |
| +4500.0·10−6 cm3/mol | |
| Structure[1] | |
| hexagonal, hP3 | |
| P3m1, No. 164 | |
α = 90°, β = 90°, γ = 120° | |
| Thermochemistry | |
| 79 J·mol−1·K−1[2] | |
Std enthalpy of formation(ΔfH⦵298) | −538 kJ·mol−1[2] |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms | [3] |
| GHS Signal word | Danger[3] |
| H302, H332, H315, H334, H317, H341, H350, H360, H372[3] | |
| P260, P284, P201, P280, P405, P501[3] | |
| Lethal dose or concentration (LD, LC): | |
| 1515 mg/kg (oral, rat) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
- However, upon addition of water this reaction was rapidly reversed. According to Tschugaeff (83) Ni(DMG)^ did not react with phenyl isocyanate; and Barker (10) could not find any reaction with acetic anhydride. Barker did claim that Ni(DMG)2 was methylated by methyl iodide; hut Thilo and Friedrich (81) reported that they observed no reaction with.
- Preparations: Ni + F 2 55°C /slow → NiF 2 Ni + Cl 2 EtOH/ 20°C → NiCl 2 Ni + Br 2 red heat → NiBr 2 NiCl 2 + 2NaI → NiI 2 + 2NaCl. Nickel carbonate usually occurs as a light green crystalline solid or a brown powder. It dissolves in ammonia and dilute acids but is insoluble in hot water.
- I did an experiment to form Ni (HDMG)2 from Ni (NH3)6 (BF4)2 and I'm asked a question to explain why the Ni (HDMG)2 is insouble from a solution of deionised water, conc HCl and Ammonium Hydroxide.
- Mar 24, 2011 Dimethylglyoxime, IUPAC name: 2,3-Butanedione Dioxime, Formula: C4H8N2O2 is a white cristalline solid, insoluble in water, but soluble in some organic solvents. The molecule has two acidic protons and thus dissolves in aqueous NaOH as a sodium salt.
Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is an apple-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.[4]
Nov 08, 2019 If you see the 'no mountable file systems error' while opening a dmg, here's what you should try: In most cases, the downloaded dmg file is actually corrupt or had an error downloading. If possible, try downloading the dmg again, turning off any download assistant plug-ins you may have. If you are facing Mac error no mountable file systems problem, follow these steps: In most cases, the downloaded dmg file is actually corrupt or had an error downloading. If possible, try downloading the dmg again, turning off any download assistant plug-ins you may have. You can try downloading the file in a different browser as well. 
Properties[edit]
Why is the NI DMG complex insoluble in water? Ni(DMG)2 is a neutral complex (Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen) that are typically insoluble because there are no charges on the complex that polar water molecules can bind to and solvate the ion.
Who we aredmg media’s brands deliver brilliant content to millions of loyal customers around the globe, 24 hours a day, seven days a week.In the UK alone, the Published Audience Measurement Company (PAMCo) released data in April 2018 showing our brands reach more adults on a daily, weekly and monthly basis than any other newsbrand. On a monthly basis, our brands reach 67% of the population, engaging with a larger audience than any other publisher.Without fear or favour, our people pride themselves on getting to the bottom of the stories most relevant to our readers. Dmg entertainment and media co ltd subsidiaries inc.
Nickel(II) hydroxide has two well-characterized polymorphs, α and β. The α structure consists of Ni(OH)2 layers with intercalated anions or water.[5][6] The β form adopts a hexagonal close-packed structure of Ni2+ and OH− ions.[5][6] In the presence of water, the α polymorph typically recrystallizes to the β form.[5][7] In addition to the α and β polymorphs, several γ nickel hydroxides have been described, distinguished by crystal structures with much larger inter-sheet distances.[5]
The mineral form of Ni(OH)2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals.[8] A nickel-magnesium variant of the mineral, (Ni,Mg)(OH)2 had been previously discovered at Hagdale on the island of Unst in Scotland.[9]
Reactions[edit]
Nickel(II) hydroxide is frequently used in electrical car batteries.[6] Specifically, Ni(OH)2 readily oxidizes to nickel oxyhydroxide, NiOOH, in combination with a reduction reaction, often of a metal hydride (reaction 1 and 2).[10]
Reaction 1 Ni(OH)2 + OH− → NiO(OH) + H2O + e−
Reaction 2 M + H2O + e− → MH + OH−
Net Reaction (in H2O)Ni(OH)2 + M → NiOOH + MH
Of the two polymorphs, α-Ni(OH)2 has a higher theoretical capacity and thus is generally considered to be preferable in electrochemical applications. However, it transforms to β-Ni(OH)2 in alkaline solutions, leading to many investigations into the possibility of stabilized α-Ni(OH)2 electrodes for industrial applications.[7]
Synthesis[edit]
The synthesis entails treating aqueous solutions of nickel(II) salts with potassium hydroxide.[11]
Toxicity[edit]
The Ni2+ ion is a known carcinogen. Toxicity and related safety concerns have driven research into increasing the energy density of Ni(OH)2 electrodes, such as the addition of calcium or cobalt hydroxides.[4]
See also[edit]
References[edit]
- ^Enoki, Toshiaki; Tsujikawa, Ikuji (1975). 'Magnetic Behaviours of a Random Magnet, NipMg(1-p)(OH2)'. Journal of the Physical Society of Japan. 39 (2): 317. doi:10.1143/JPSJ.39.317.
- ^ abZumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN978-0-618-94690-7.
- ^ abcd'Nickel Hydroxide'. American Elements. Retrieved 2018-08-30.
- ^ abChen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. (1999). 'Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries'. J. Electrochem. Soc. 146 (10): 3606–3612. doi:10.1149/1.1392522.
- ^ abcdOliva, P.; Leonardi, J.; Laurent, J.F. (1982). 'Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides'. Journal of Power Sources. 8 (2): 229–255. doi:10.1016/0378-7753(82)80057-8.
- ^ abcJeevanandam, P.; Koltypin, Y.; Gedanken, A. (2001). 'Synthesis of Nanosized α-Nickel Hydroxide by a Sonochemical Method'. Nano Letters. 1 (5): 263–266. doi:10.1021/nl010003p.
- ^ abShukla, A.K.; Kumar, V.G.; Munichandriah, N. (1994). 'Stabilized α-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells'. J. Electrochem. Soc. 141 (11): 2956–2959. doi:10.1149/1.2059264.
- ^Marcopoulos, T.; Economou, M. (1980). 'Theophrastite, Ni(OH)2, a new mineral from northern Greece'(PDF). American Mineralogist. 66: 1020–1021.
- ^Livingston, A.; Bish, D. L. (1982). 'On the new mineral theophrastite, a nickel hydroxide, from Unst, Shetland, Scotland'(PDF). Mineralogical Magazine. 46 (338): 1. doi:10.1180/minmag.1982.046.338.01.
- ^Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. (1993). 'A nickel metal hydride battery for electric vehicles'. Science. 260 (5105): 176–181. doi:10.1126/science.260.5105.176. PMID17807176.
- ^Glemser, O. (1963) 'Nickel(II) Hydroxide' in 'Handbook of Preparative Inorganic Chemistry, 2nd ed. G. Brauer (ed.), Academic Press, NY. Vol. 1. p. 1549.
External links[edit]
Why Is Ni Dmg 2 Insoluble In Water System
| Names | |
|---|---|
| IUPAC name | |
Other names
| |
| Identifiers | |
| |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.201 |
| EC Number | |
PubChemCID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C4H8N2O2 | |
| Molar mass | 116.120 g·mol−1 |
| Appearance | White/Off White Powder |
| Density | 1.37 g/cm3 |
| Melting point | 240 to 241 °C (464 to 466 °F; 513 to 514 K) |
| Boiling point | decomposes |
| low | |
| Structure | |
| 0 | |
| Hazards | |
| Main hazards | Toxic, Skin/Eye Irritant |
| Safety data sheet | External MSDS |
| GHS pictograms | |
| GHS Signal word | Danger |
| H228, H301 | |
| P210, P240, P241, P264, P270, P280, P301+310, P321, P330, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| Hydroxylamine salicylaldoxime | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
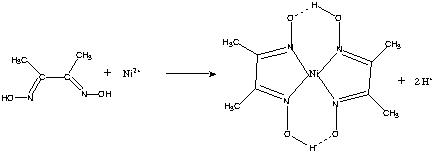
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.
Ni Dmg 2 Complex
Preparation[edit]
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:[1]
Complexes[edit]
Dimethylglyoxime is used to detect and quantify nickel, which forms the bright red complex nickel bis(dimethylglyoximate) (Ni(dmgH)2). The reaction was discovered by L. A. Chugaev in 1905.[2]
Cobalt complexes have also received much attention. In chloro(pyridine)cobaloxime[3] the macrocycle [dmgH]22− mimics the macrocyclic ligand found in vitamin B12.
References[edit]

Why Is Ni Dmg 2 Insoluble In Water Heater
- ^Semon, W. L.; Damerell, V. R. (1930). 'Dimethylglyoxime'. Organic Syntheses. 10: 22. doi:10.15227/orgsyn.010.0022.CS1 maint: multiple names: authors list (link)
- ^Lev Tschugaeff (1905). 'Über ein neues, empfindliches Reagens auf Nickel'. Berichte der Deutschen Chemischen Gesellschaft. 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
- ^Girolami, G. S.; Rauchfuss, T.B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual (3rd ed.). pp. 213–215.